Page 122 WHO - Guidelines on the pharmacological treatment of persisting pain in children with medical illness
P. 122
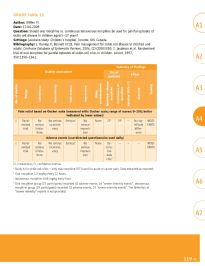
GRADE Table 13
Author: Wiffen PJ A1
Date: 17-04-2009
Question: Should oral morphine vs. continuous intravenous morphine be used for painful episodes of
sickle cell disease in children aged 5–17 years?
Settings: Jacobson study: Children’s hospital, Toronto, ON, Canada.
Bibliography: 1. Dunlop R, Bennett KCLB. Pain management for sickle cell disease in children and
adults. Cochrane Database of Systematic Reviews, 2006, (2):CD003350; 2. Jacobson et al. Randomised
trial of oral morphine for painful episodes of sickle-cell crisis in children. Lancet, 1997,
350:1358–1361. A2
Summary of findings
Quality assessment No. of
patients Effect
No. of studies Design Limitations Inconsistency Indirectness Imprecision Other considerations Modified- release morphine Continuous IV morphine Relative (95% CI) Absolute Quality A3
Pain relief based on Oucher scale (measured with: Oucher scale; range of scores: 0–100; better
indicated by lower values)
1 Rand- No No serious Serious a No None 27 b 29 c – No sig- MOD- A4 A4
omized serious inconsist- serious nificant ERATE
trial limita- ency impreci- differ-
tions sion ence
Adverse events (non-directed questionnaire used daily)
1 Rand- No No serious Serious a No None De- – – – MOD-
omized serious inconsist- serious scrip- ERATE
trial limita- ency impreci- tive A5
tions sion data
only d
IV, intravenous; CI, confidence interval.
a Study is for sickle cell crisis – only oral morphine RCT found for acute or cancer pain. Data extracted as reported.
b Oral morphine 1.9 mg/kg every 12 hours.
c Intravenous morphine 0.04 mg/kg every hour. A6
d Oral morphine group (27 participants) recorded 62 adverse events, 16 “severe intensity events”. Intravenous
morphine group (29 participants) recorded 52 adverse events, 19 “severe intensity events”. The definition of
“severe intensity” reports is not provided.
A7
119 <