Page 123 WHO - Guidelines on the pharmacological treatment of persisting pain in children with medical illness
P. 123
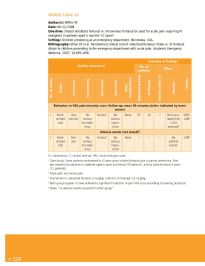
GRADE Table 14
Author(s): Wiffen PJ
Date: 08-12-2008
Question: Should nebulized fentanyl vs. intravenous fentanyl be used for acute pain requiring IV
analgesics in patients aged 6 months–17 years?
Setting: Children presenting at an emergency department, Minnesota, USA.
Bibliography: Miner JR et al. Randomized clinical trial of nebulized fentanyl citrate vs. IV fentanyl
citrate in children presenting to the emergency department with acute pain. Academic Emergency
Medicine, 2007, 14:895–898.
Summary of findings
Quality assessment No. of
patients Effect
No. of studies Design Limitations Inconsistency Indirectness Imprecision Other considerations Nebulized fentanyl IV fentanyl Relative (95% CI) Absolute Quality
Reduction in VAS pain intensity score (follow-up: mean 30 minutes; better indicated by lower
values)
1 Rand- Very No Serious b No None 27 c 14 – Not calcu- VERY
omized serious a serious serious lated Only LOW
trial inconsist- impre- 11/41
ency cision assessed d
Adverse events (not stated )
d
1 Rand- Seri- No Serious b No None – – – No LOW
omized ous a serious serious adverse
trial inconsist- impre- events e
ency cision
IV, intravenous; CI, control interval; VAS, visual analogue scale.
a Open study. Some patients randomized to IV were given inhaled fentanyl due to parent preference. Pain
was assessed by physician in patients aged 6 years and below (30 patients), and by patients above 6 years
(11 patients).
b Acute pain not cancer pain.
c Intervention is nebulized fentanyl 3 mcg/kg; control is IV fentanyl 1.5 mcg/kg.
d Both groups appear to have achieved a significant reduction in pain VAS score according to treating physician.
e States “no adverse events occurred in either group”.
> 120